
PolyNovo develops innovative medical devices utilizing the patented bioabsorbable polymer technology NovoSorb®.
NovoSorb is a family of proprietary medical grade polymers that can be expressed in a variety of physical formats.
NovoSorb polymers have advantageous properties such as biocompatibility and design flexibility.
These properties underpin novel medical devices designed to support tissue repair, before they biodegrade in situ into biocompatible by-products via established pathways.
NovoSorb BTM (Biodegradable Temporizing Matrix) is the first product commercialized by PolyNovo. In 2015, following the FDA 510(K) clearance of BTM, PolyNovo was awarded an $11 million contract and a further $25 million in funding from the US-based Biomedical Advanced Research and Development Authority (BARDA) to complete a trial with NovoSorb BTM for full thickness burns.
PolyNovo has expanded their manufacturing facility and headquarters in Melbourne, Australia, establishing high quality processes, enabling product innovation and preparing for growth.
NovoSorb® BTM (Biodegradable Temporizing Matrix) is a synthetic, biodegradable and biocompatible device designed to facilitate the dermis to grow within a patented polyurethane matrix.
When ready, the sealing membrane is removed, leaving a fully vascularized dermis, ready for definitive closure.

Biocompatible polymers for novel medical devices
These biocompatible polymers are designed to support different functions of the body and then biodegrade into by-products that can be absorbed and excreted by the body.
The patented NovoSorb polymers can be produced in a range of formats with different mechanical and degradation properties. In addition to NovoSorb BTM, they can be used for thermoplastic extrusions, filaments for weaving or knitting and as a solution for spray or dip coatings of other devices. This versatility leads to a promising product pipeline for PolyNovo.
 .
. 

Leveraging synthetic materials, rather than biologic, means there is an absence of foreign sensitizing proteins, which can help reduce the risk of rejection. Most importantly, because NovoSorb does not contain any biologic material to feed bacterial infection, NovoSorb devices have been shown to be robust in the presence of infection1.
Key Attributes of NovoSorb:
PolyNovo has no royalty or license obligations to any other parties. NovoSorb BTM is the first commercially available NovoSorb product.

The difference is NovoSorb BTM:
Indications
NovoSorb® BTM is indicated for use in the management of wounds including: partial and full thickness wounds, pressure ulcers, venous ulcers, diabetic ulcers, chronic and vascular ulcers, surgical wounds (donor sites/grafts, post-Moh’s surgery, post-laser surgery, podiatric, wound dehiscence), trauma wounds (abrasions, lacerations, second-degree burns, and skin tears) and draining wounds.
Phases of NovoSorb BTM Integration
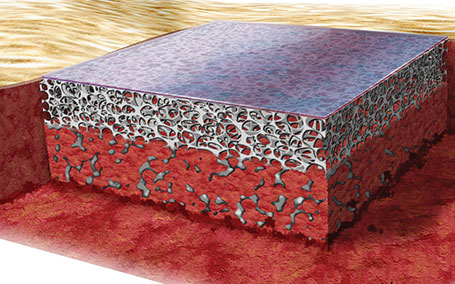
1. NovoSorb® BTM implanted into a surgically debrided wound bed
The wound is physiologically closed, limiting the risk of infection, evaporative moisture loss and contraction1,2.

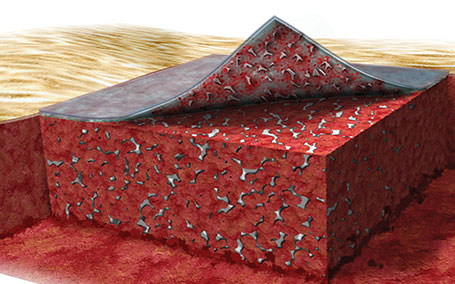
2. Integration process of NovoSorb® BTM
Over a period of approximately 2 to 3 weeks, NovoSorb® BTM integrates into the wound bed through cellular infltration.
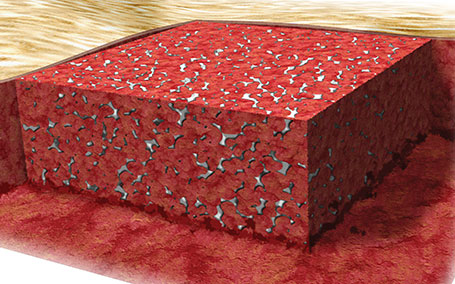
3. NovoSorb® BTM fully integrated
The dermis is regenerated within the matrix. Once fully integrated, the sealing membrane is ready for removal.

4. Sealing membrane removed
Once the sealing membrane is removed the neodermis is ready for secondary treatment.
5. Secondary treatment
Method of secondary treatment is left to the physician’s clinical choice (e.g. closure by SSG, or closure by secondary intent). The NovoSorb® BTM progressively biodegrades and is fully absorbed in approximately 18 months7.
Converting wound repair into regeneration
NovoSorb BTM compartmentalizes a large wound into a series of interconnected microwounds. The body easily heals microwounds, promoting organized regenerative healing.
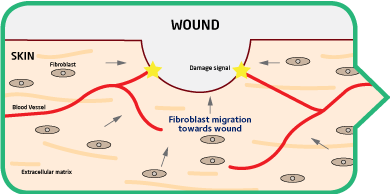

Normal Healing Healing with NovoSorb BTM
The body’s natural reparative process follows the chaotic, NovoSorb BTM provides a unique matrix for organized healing.
unorganized laying down of fibrotic tissue in order to rapidly Cells and blood vessels migrate into the
close the wound. This is followed by months of remodeling NovoSorb BTM and a new vascularized
and scar contraction. dermal-like structure is formed5.
The body heals each chamber as a
discrete small wound8.
Clinical Application
NovoSorb® BTM is convenient to store and prepare for application:
Two key stages with NovoSorb BTM:
Application
Once the wound is prepared, NovoSorb BTM is stapled or sutured in place with the device flush against the edges of the wound and the NovoSorb BTM laid flat across the wound bed without creases. The NovoSorb BTM is left in place with regular overlying dressing changes.
Delamination
Once capillary refll is seen throughout the NovoSorb BTM, the sealing membrane is delaminated with the top layer detaching with a ‘Velcro®-like’ action.